
Sleep Apnea Study 2
Sleep apnea study you can do from home
Apply to enroll in the Sleep Apnea Study #2 to help test an investigational technology that helps manage obstructive sleep apnea (OSA) help_outline Obstructive sleep apnea is a sleep disorder that can cause intermittent airflow blockage during sleep.
The journey from identification to successful, proven treatment of sleep apnea can be long and lonely. The more we can learn about people who snore and have poor sleep, the better we can understand how to improve diagnosis and treatment pathways that lead to better sleep, and ultimately improved health and well being

Project Baseline is an initiative to make it easy and engaging for people like you to contribute to the map of human health and participate in clinical research.
Together with researchers, clinicians, engineers, designers, advocates, and volunteers, we're collaborating to build the next generation of healthcare tools and services.




Could you have obstructive sleep apnea (OSA)?
If you snore loudly or still feel tired after a full night of sleep, you may suffer from obstructive sleep apnea (OSA). OSA is a disorder in which breathing stops and starts during sleep and it is the most common form of sleep apnea. OSA is one of many sleep disorders that are underdiagnosed.
Why we need you for this study?
We are on a mission to improve treatment technologies for OSA. We are seeking people who potentially have OSA to test a new app and PAP device and share valuable feedback about your experience.
Who's eligible
- Age 18 years or older
- US resident living in NC or TX
- Able to speak and read English
- At risk of obstructive sleep apnea (OSA)
- Owner and primary user of a smartphone
- Owner of a computer with a web camera
- Not pregnant or planning to become pregnant during the study period
- Not a worker with a regularly scheduled night shift
What's involved
Help researchers better understand your gut health by:
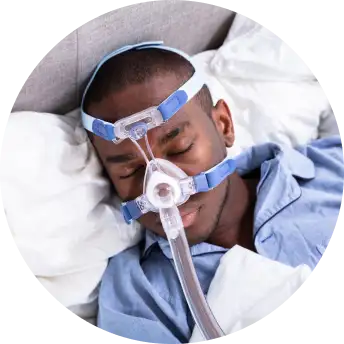
Testing investigational technology
You'll be evaluated for a home sleep test, and if diagnosed with OSA, use a mobile app and a positive airway pressure (PAP) study device for OSA management for 90 days.

Taking 5 surveys
You'll answer questions about your study experience and give feedback on the app and device.

Joining our Baseline community
Get first access to future sleep-related studies.
Sleep Apnea Study 2
Study FAQs
The most common form of treatment today for Obstructive Sleep Apnea (OSA) is the use of a positive airway pressure (PAP) device. OSA is a condition in which the upper airway collapses during sleep, preventing the air from getting into your lungs. A PAP device is used in treatment to pump air under pressure into the airway of the lungs. This helps keep the windpipe open during sleep. The forced air delivered by PAP prevents episodes of airway collapse that block the breathing in people with OSA and other breathing problems.
ResMed:
It is a ResMed Airsense10 Autoset machine:
https://www.resmed.com/us/en/consumer/products/devices/airsense-10-autoset.html
Yes.
The PAP machine arrives pre-set according to the prescription determined by the managing physician. You may be able to manually adjust your PAP machine pressure setting, however you should follow the recommendations/settings provided by the Durable Medical Equipment (DME) provider when using your machine. If these settings do not work for you, please work directly with the DME provider for adjustments.
The PAP machine works on cellular data, but doesn't use your cellular plan, so it doesn't use up your data.
The Study app uses WiFi when available or cellular data when WiFi is not available.
Please follow the instructions in the Study app unless otherwise instructed by your physician.
The study Sponsor (Verily) will stop retrieving data from your PAP device after the 90 day collection period. After the 90 day collection period, the PAP machine will continue to upload data to ResMed, but Verily will elect not to retrieve the data.
As a participant, you will have the opportunity to go through the Obstructive Sleep Apnea (OSA) diagnostic pathway and use a PAP device (if prescribed) at no cost.
The only things that should have to be replaced during the first 90 days are the cushions and filters; the Welcome Kit that is shipped for the study should have a 90 day supply of disposable filters and cushions for the duration of the study.
No, a signature is not required for receipt of the package.
When you log into the Study app with your Google account, Verily will only have access to (1) your Google profile information (specifically, your name and mail address), and (2) logs of any activity you do within the Study app. Verily will not have access to any other information in your Google accounts.
Payments will be processed using a third party (which means they are independent from the study Sponsor) payment vendor called Hyperwallet.
For a breakdown of payments, please refer to the 'Payment for Participation' section of the Informed Consent Form. Once you have signed the Informed Consent Form and completed the remote screening activities, you will be sent an email from Hyperwallet with instructions to create an account. The terms and information needed to create an account and receive payment are listed in the informed consent form.
Payments should be available in your account within 1 week of completing a task, but it may take up to 5 weeks, as statedin the Informed Consent Form.